최근 쿠싱병 의심되는 분이 있어 HDDST 를 시행하는데, Classic test 와 8mg overnight DST 중에 어떤 것이 정확한지 의문이 들어 자료를 찾아보던 중 좋은 내용이 있어 발췌한 내용이다.
Cushing Disease
John D.C Newell-Price, in The Pituitary (Fourth Edition), 2017
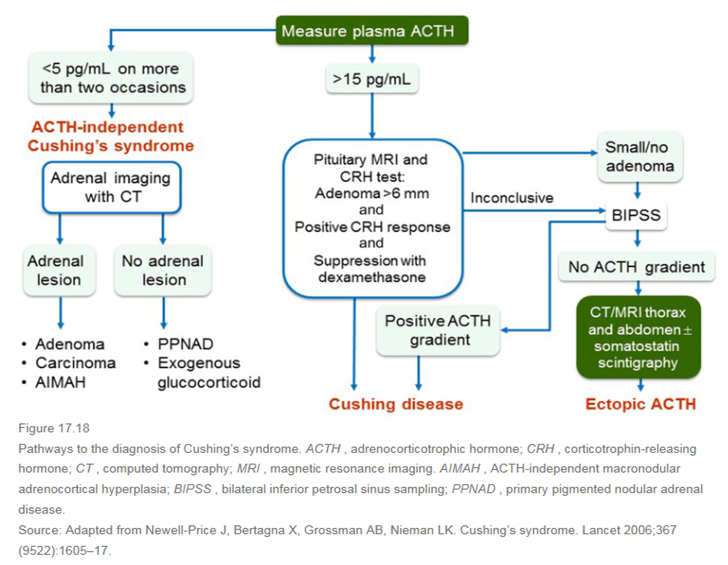
쿠싱증후군과 관련하여 가장 중요한 표이다.
Cortisol 과다 상태가 확인이 되면 그 Cortisol 이 어디에서 나오는지를 생각해야 하겠다. ACTH 가 중요한 지표가 될 것인데, ACTH 가 억제되어 있다면 adrenal adenoma 는 아닌지 CT 를 촬영해봐야 할 것이고, ACTH 도 많이 나오고 있다면 ACTH-dependent Cushing’s syndrome 중에 머리 문제인지, Ectopic 인지 감별해야 한다. 3가지 검사를 더 하자. Sella MRI, High dose Dexamethasone suppression test, CRH stimulation test 이다. High dose DST 를 하는 이유는, high dose 로 사용하였을때 비로소 CRH 가 억제되기 때문이며, CRH stimulation 의 경우에 쿠싱병이라면 Cortisol 과 ACTH 수치가 기저치보다 증가하게 된다. 이러한 검사에서 양성이면 Cushing’s disease 라고 생각할 수 있겠지만 애매하다면 침습적인 IPSS 를 하여 Ectopic ACTH syndrome 을 감별해야 하겠다. (Sella MRI 를 촬영하여 adenoma 가 있다고 해도, incidentaloma 의 가능성도 있으므로!)
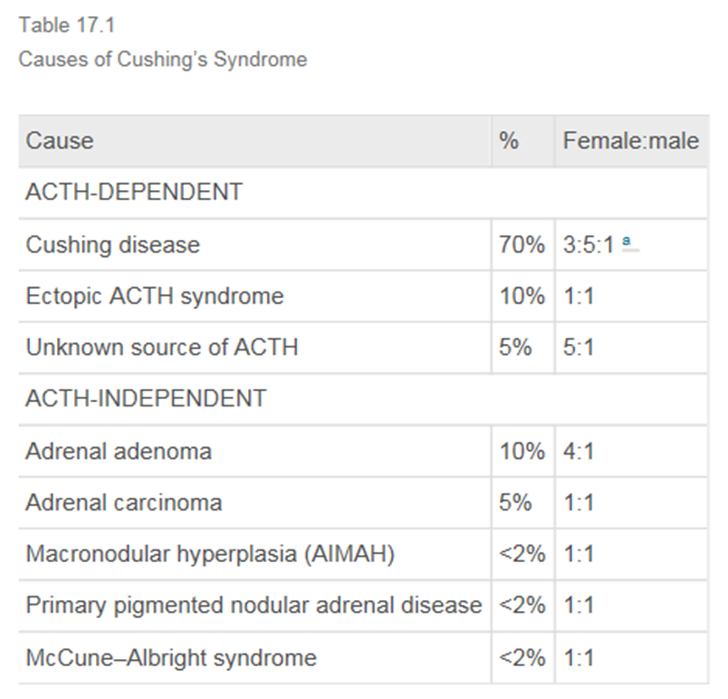
Cushing’s syndrome 에서 Cushing’s disease 의 비율이 제일 많다. 그래서 Cushing’s diease 로 지어지게 되었나보다. 그 다음 Ectopic ACTH syndrome 으로 생각보다 많다.
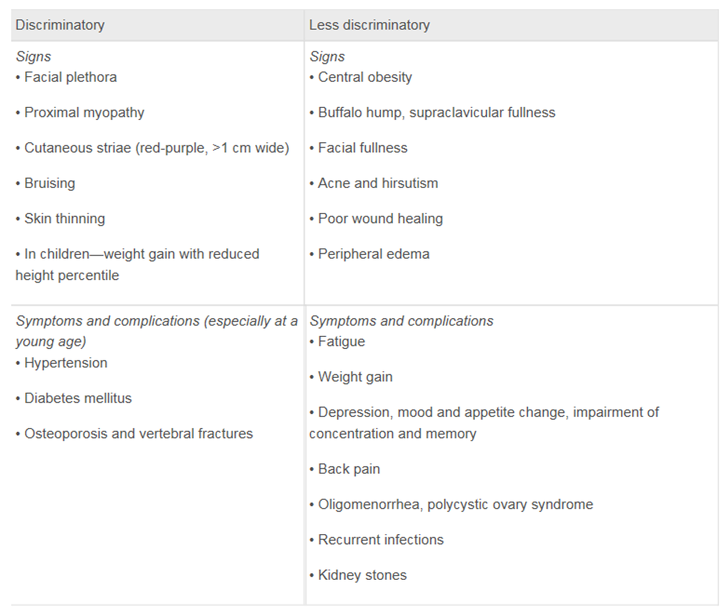
쿠싱병이 알려지면서 배만 나오고, 얼굴만 크면 다 쿠싱아니냐고 한다. 도움이 될만한 표이다.
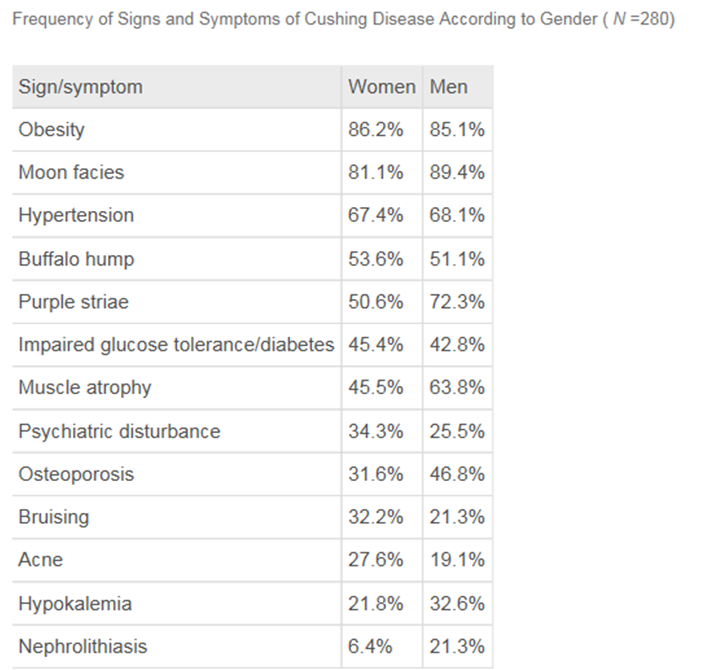
Buffalo hump 가 없을 수도 있고, Purple striae 가 없을 수도 있다.
Establishing the Pituitary Origin of the ACTH-Driven Hypercortisolemic State
Once it is established that the patient has ACTH-dependent Cushing’s syndrome, the next step is to determine if the source is of pituitary (Cushing disease) or nonpituitary (ectopic ACTH syndrome) origin. These may be divided into noninvasive baseline and dynamic tests and invasive dynamic tests.
Noninvasive Baseline Tests
(1) Plasma ACTH levels.
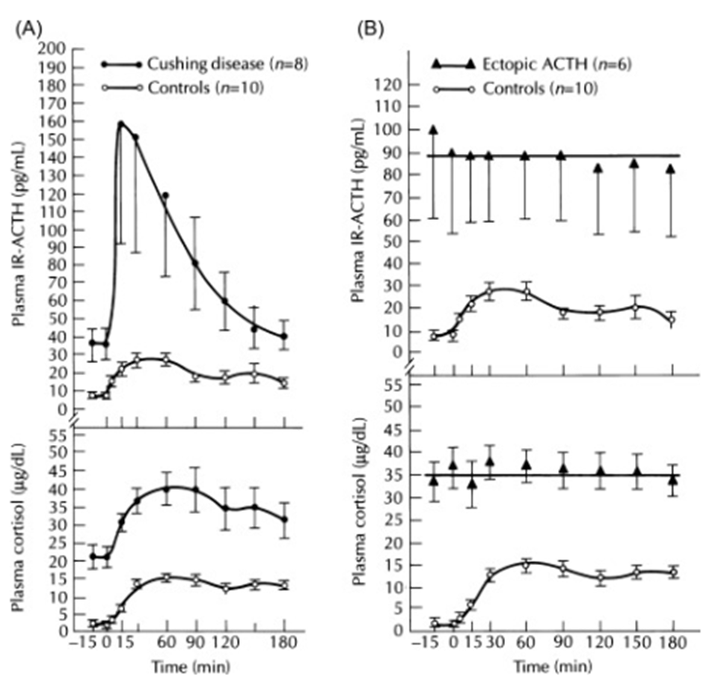
Patients with ectopic ACTH syndrome tend to have higher levels than patients with Cushing disease, although there is considerable overlap between the two groups[25327347] and further investigations are needed for a clear separation ( Fig. 17.14 ). If the ACTH levels are very high an ectopic source is more likely.
(3) Plasma non-ACTH POMC peptides.
Altered POMC processing is common in patients with nonpituitary tumors, but is normal in most pituitary corticotroph microadenomas[2224] . This difference has been suggested as a way of differentiating pituitary and nonpituitary sources of ACTH by using multitargeted IRMA assays that recognize abnormal POMC processing at a given location on the precursor molecule[356357] . These approaches have their limitations, as they are only available to highly specialized laboratories, and sensitivity is low and specificity not perfect. Rare cases of pituitary macroadenoma have been described which do not process POMC normally[259697] and these would be classified as “nonpituitary” by these tests. For these reasons these tests are not in widespread use.
(3) Plasma potassium.
Hypokalemic alkalosis is present in more than 95% of patients with the ectopic ACTH syndrome but in fewer than 10% of those with Cushing disease. The explanation is that patients with the ectopic syndrome usually have higher rates of cortisol secretion. Cortisol saturates the renal-protective HSD11B2 enzyme, resulting in cortisol-induced mineralocorticoid hypertension. In addition, these patients have higher levels of the ACTH-dependent mineralocorticoid, DOC.
(4) Tumor markers.
Some tumors that cause ectopic ACTH secretion also secrete other tumor markers, which may be of use: pheochromocytomas (urinary and plasma-free normetanephrine and normetanephrine); medullary thyroid carcinoma (calcitonin); and gastrinomas (gastrin). Chromogranin A had a positive predictive value of 83% and a negative predictive value of 70% for the diagnosis of EAS in a study on six patients with occult ectopic ACTH secretion diagnosed by central venous catheterization, 11 patients with histologically proven ectopic ACTH secretion, and 25 patients with Cushing disease[358] .
Dynamic Noninvasive Testing
(1) The classic 48-hour high-dose dexamethasone suppression test (HDDST).
The rationale for the HDDST is that, in Cushing disease, negative feedback control of ACTH is reset to a higher level than normal. Therefore, cortisol levels do not suppress with low-dose dexamethasone but do so after high doses. The original test introduced by Liddle was based on giving 2 mg dexamethasone every 6 hours for 48 hours and demonstrating a fall of greater than 50% in urinary 17-hydroxycorticosteroids[55] . In the modern test, serum cortisol is measured at 0 and +48 hours, and a greater than 50% suppression of plasma cortisol from the basal value has been used to define a positive response. Numerous series have confirmed that whereas some 60–85% of patients with Cushing disease suppress to less than 50% of baseline values on the high-dose dexamethasone test, no rigid cutoff excludes the diagnosis of Cushing disease[11327] . The specificity can be improved using a cutoff of cortisol suppression greater than 90%, although a specificity of 100% is never attained[11359] .
(2) The overnight 8 mg HDDST.
In this test, 8 mg of dexamethasone is given orally as a single dose at 23:00 or 24:00 hours and the 08:00 hours or 09:00 hours serum cortisol the next morning is compared with that of the previous (control) day. The proposed cutoff point for a positive response (suppressibility) is a serum cortisol decrease to 50% or less of its control value[360] . With this arbitrary criterion, two studies gathering 73 patients with Cushing disease, 8 with adrenocortical tumors, and 12 with ectopic ACTH syndrome compared this overnight test directly with the classic HDDST performed in the same patients. This test appeared at least as efficient if not better, with 89% sensitivity and 100% specificity for diagnosis of Cushing disease[361] . That this overnight suppression test reaches higher diagnostic accuracy than the classic test may be explained by the fact that the 8 mg dose is given as a single administration. In the same manner as for the classic test, there is no theoretical reason to fix a rigid cutoff at a 50% decrease.
Overall, the role of any form of the HDDST in modern practice can be questioned. In women with ACTH-dependent Cushing’s syndrome, where the a priori likelihood of Cushing disease is 90%, the sensitivity of this test for Cushing disease is 80%, which is lower than the pretest probability. In addition, around 10% of patients with ectopic ACTH syndrome show suppression of serum cortisol >50%[240327362] . Because of this there is little logic to the continued use of this test where IPSS is available (see below). Moreover, if the LDDST has been used in the diagnosis of Cushing’s syndrome and a >50% fall in cortisol is observed, there is no added value in the HDDST[363] .
(3) The metyrapone test.
Metyrapone testing has been superseded by other tests, and there are few reasons to use this test in the differential diagnosis of ACTH-dependent Cushing’s syndrome.
Direct assessment of pituitary ACTH reserve. Several secretagogues that act specifically on the corticotroph cell in the normal subject have been used in Cushing’s syndrome with the assumption that they would only trigger further ACTH secretion if the latter were of pituitary origin. This premise is questionable as the receptors responding to the secretagogues are not invariably expressed only in corticotroph tumors and some ectopic ACTH-secreting tumors may respond.
(4) The desmopressin test.
Desmopressin (1-deamino-8D-arginine vasopressin), a V2 and V3 (V1b) agonist, has been used as an ACTH secretagogue in Cushing disease, with few side effects. Response is assessed by measuring ACTH, serum cortisol, and possibly salivary cortisol, at various intervals—30 minutes before, basally, 15, 30, 45 and 60 minutes after intravenous administration of 10 μg of desmopressin, with serial blood samples obtained from an indwelling catheter inserted in a forearm vein. Patients have to restrict fluid intake for the remainder of the day to avoid water overload. Adverse effects are limited to short-lived flushing sensation, transient tachycardia, mild and transient decrease in blood pressure, headache, abdominal pain, or weight increase. There are several criteria for interpretation in the literature (parameter: ACTH or cortisol; threshold: a relative increase of more than 35–50% in ACTH or of more than 20–36% in cortisol)[11] . The desmopressin test induces a positive ACTH response in approximately 85% of patients with Cushing disease[364365] . Unfortunately, the V3 receptor is expressed in as many as 30% of ectopic tumors secreting ACTH[364366] , limiting the usefulness of the desmopressin test in the differential diagnosis of ACTH-dependent Cushing’s syndrome. It might prove more useful in the postoperative assessment to predict recurrence after pituitary surgery as normal subjects rarely respond to the test[11367368] .
(5) The CRH test.
A major breakthrough was achieved with the isolation, characterization, and synthesis of ovine CRH in 1981[33] . This discovery opened up new avenues to investigators of corticotroph function in humans[369370] . Thorough studies in normal subjects showed that the ovine peptide was active in humans, eliciting an ACTH response definitively higher than that elicited by LVP, but still lower than that obtained after insulin-induced hypoglycemia[371372] . Dose–response studies indicated that administration of 10 μg/kg body weight maximally stimulated cortisol release, while larger amounts could induce still higher ACTH responses[371] .
Synthetic ovine-sequence CRH (in many countries including the US) or synthetic human sequence CRH (in Europe) is administered intravenously at a dose of 100 μg in adults or 1 μg/kg body weight in children. Response is assessed by measuring ACTH, serum cortisol, and possibly salivary cortisol levels 30 minutes before, baseline, 15, 30, 45 and 60 minutes after administration of CRH with serial blood samples obtained from an indwelling catheter inserted in a forearm vein. The test is well tolerated; the few side effects are mild facial flushing, a metallic taste in the mouth, and neck tightness. It is usually performed in the morning (09:00 hours). Data comparing ovine and human CRH peptides in the same patients confirm that the former has a higher potency and provides a better diagnostic accuracy, largely outweighing the theoretical advantage of using a homologous peptide, at least for a single-dose testing[130] .
The CRH test relies on it being a potent and specific stimulator of pituitary ACTH, whereas tumors causing an ectopic ACTH syndrome rarely respond ( Fig. 17.16 )[130] .
Confusion may arise with the various ways different authors not only administer CRH but also assess an “exaggerated” or a “flat” response[369] . Using data from 10 published series, Kaye and Crapo[360] developed their own criteria from the combined data: a positive response being an increase of more than 50% in ACTH or of more than 20% in cortisol, with a sensitivity and specificity of 80% and 95%, respectively, using the ACTH response, and 91% and 95%, respectively, using the cortisol response. There is general agreement that the test has a high diagnostic accuracy, which compares favorably with that of the classic HDDST. In evaluating 100 patients with Cushing disease and 16 patients with ectopic ACTH secretion, after a single a.m. CRH stimulation, 7% of patients with ACTH-dependent Cushing’s did not respond, while no patient with ectopic ACTH secretion responded to CRH[373] . A positive response is highly suggestive of Cushing disease, and the stronger the response the higher the probability. In another study with 101 patients with Cushing disease and 14 patients with ectopic ACTH secretion, the sensitivity and specificity of the human CRH test for the diagnosis of Cushing disease were 70% and 100%, respectively, using a relative increase of more than 105% in ACTH, and 85% and 100%, respectively, using a relative increase of more than 14% in cortisol response[374] .
The stimulatory action of CRH can be strengthened by the synergistic effect of AVP or its V1 analogues[375] . Data obtained with a combined CRH/AVP test showed that all patients with Cushing disease responded positively[376] . A desmopressin-CRH test may be more discriminatory, with a specificity of 80–100%[377] , but is rarely used.
Occasional patients with ectopic ACTH syndrome exhibited an apparent positive response[240] . These rare cases may lead to unwarranted pituitary surgery for a mistaken diagnosis of Cushing disease, emphasizing the role of bilateral IPSS (see below).
Concordant positive responses to CRH and dexamethasone testing make Cushing disease highly likely.
Invasive Testing: Bilateral Inferior Petrosal Sinus Sampling
The most accurate test to distinguish Cushing disease from the ectopic ACTH syndrome is bilateral IPSS. As blood from each half of the pituitary drains into the ipsilateral inferior petrosal sinus, catheterization and venous sampling of both sinuses simultaneously can distinguish a pituitary from an ectopic source of ACTH. The goal of this approach is to establish whether ACTH oversecretion is of pituitary or nonpituitary origin. Both sinuses and peripheral blood must be sampled simultaneously. Discomfort around the ears is frequently reported during the test and patients need to be warned about the flushing sensation induced by CRH. This investigation should only be considered and performed in centers where there is a great deal of experience.
A central-to-peripheral ACTH gradient is calculated by the ratio of ACTH plasma levels in the inferior petrosal sinus with the highest level over ACTH in the peripheral blood, before and after stimulation (measured at 2, 5, 10 minutes), usually with CRH 100 µg IV. A basal central:peripheral ratio of >2:1 or a CRH stimulated ratio of >3:1 is indicative of Cushing disease. Analysis of different series and individual case reports confirm the diagnostic power of the procedure to discriminate between Cushing disease and ectopic ACTH syndrome with a sensitivity and a specificity of 94%378379380381382383384385 . In virtually all patients with the ectopic ACTH syndrome, the ratio of ACTH concentration in the inferior petrosal sinus and that in simultaneously drawn peripheral venous blood is less than 1.4[385] . Where CRH is not available, some centers use desmopressin as a secretagogue, but central-to-peripheral gradients have been observed in some patients with ectopic ACTH when using this peptide.
The main causes of false-negative test results are IPSS that is not sufficiently selective, plexiform vascularization in at least one sinus, abnormal venous drainage of an adenoma that is intrasphenoidal but not strictly intrasellar, and intermittent ACTH secretion in a case of Cushing disease. Catheter position should be checked by venous angiograms[386] . The main causes of false-positives are investigation done during a period of normal cortisol levels in the presence of a tumor causing ectopic but intermittent ACTH secretion, and ectopic tumor secretion of CRH[387] . When a negative gradient is found and venograms confirm correct catheter placement, measurement of prolactin in the samples and correction of the ACTH values can reduce the false-negative rate in Cushing disease[386388389] .
Although an invasive test, particularly considering the general vascular fragility of patients with Cushing’s syndrome, the frequency of serious side effects is low. Venous thromboembolism, sixth nerve palsy, venous subarachnoid hemorrhage, and brain stem infarction have been reported390391392393 .
Some have proposed cavernous sinus[394] or jugular venous sampling395396397 and some substitute CRH by desmopressin, without major adverse effects during or after the procedure[398399] , but these approaches are less widespread.
In addition to confirming a central source of ACTH, bilateral IPSS has been evaluated as a means to localize the pituitary microadenoma within the gland, by evaluating the sinus-to-sinus ACTH gradient[400401] . Unfortunately, the test has a lateralization accuracy of 70% at best, and although successful blind hemihypophysectomies directed by sampling lateralization have been claimed[401402] , this approach is not generally recommended.