Molecular anatomy of AKI subtypes
injured tubule 의 molecular changes 를 밝혀내는 것이 understanding the processes that
underlie kidney damage 에 중요한 열쇠일 것이다.
저자가 밝혀낸 것들의 예시.
Stimuli 에 영향을 받는 dominant regions
Volume depletion predominantly affected the inner medulla
ischaemia targeted the outer strip of the outer medulla
영향을 주는 non- dominant segments 도 있더라.
ischaemia- induced RNA expression of Havcr in the proximal tubule and Lcn2 in the distal nephron
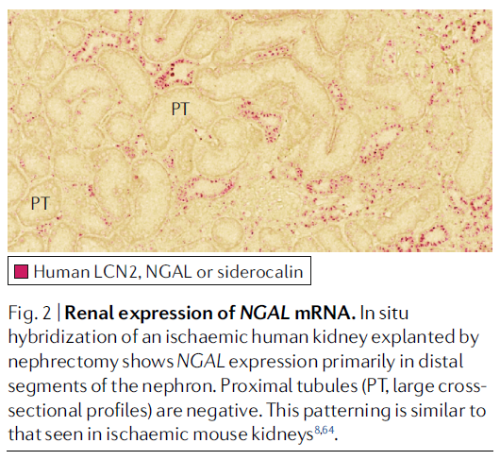
Anatomical evidence for injury subtypes
1) Toxin- induced tubular injury
Several toxins injure very specific segments of the nephron
Ex.1 ) myoglobin –> filtered through the glomerulus –> reabsorbed from the ultrafiltrate via the endocytic receptors megalin and cubilin in the proximal tubule –> death of the
epithelia 를 유발.
Ex.2 ) cadmium –> reabsorbed from glomerular ultrafiltrate via megalin –> injures the proximal tubule
– Rat 의 경우 cadmium- induced injury 때 Cell death 전, 단백뇨 시작 4~5주 전 urine KIM1 expression 이 증가
Ex.3 ) aminoglycosides –> accumulate within proximal epithelia via megalin –> cell death 유발
– gentamicin 의 경우에도 SCr 변화가 없어도 urine KIM1 이 증가
– 따라서 KIM1 은 gentamicin- induced acute tubular injury 를 더 빠르게 진단할 수 있는 좋은 marker 가 될 수 있겠다. (SCr 보다도)
– 현재 detection of kidney tubular injury 를 위한 biomarker panel (6가지 : clusterin, cystatin- C, KIM1, NAG, NGAL and osteopontin) 가 FDA 의 승인을 받았고 phase I clinical trial 중이다.
2) Sepsis- associated tubular injury
Anatomical characterization of sepsis- associated tubular injury has not yet been elucidated
Rat : lipopolysaccharide (LPS) injection –> accumulation of the endotoxin strictly within proximal tubules within 60 min, without uptake of LPS in distal tubules.
Uptake of LPS correlates with changes in the distribution of Toll- like receptors (TLRs)
TLR4 is primarily expressed in the distal tubules of healthy mice, but its expression is dramatically increased in proximal tubular cells following colon puncture.
Sepsis induced the expression of the TLR4 co- receptor CD14
CD14 expression was significantly upregulated in proximal tubules during sepsis
3) Ischaemia–reperfusion injury
the last (S3) segment of the proximal tubule & the medullary thick ascending limb (mTAL)Both segments
–> located in the medulla –> susceptible to ischaemic insults
warm IRI generates a proximal (S3) pattern of injury
반면 mTAL injury (distal nephron models) <– triggered by : cold ischaemia, vasoconstrictors, contrast agents, Gram negative sepsis, myoglobinuria and obstruction
Proximal tubule : aerobic oxidative metabolism –> continuous supply of oxygen 필요하다.
반면, mTAL : capable of some degree of anaerobic metabolism
mTAL damage 의 경우 transport activity 가 지배적인 energy-consuming process 이다. 따라서 transport inhibition might afford protection to this segment.
Secondary effects
IRI 의 secondary effects 는 현상을 해석하는 것을 복잡하게 만든다.
Secondary effects : mediated by the hypoxia- induced expression of genes
– hypoxia-inducible factor (HIF) 에 의해 조절 –> local microcirculation 을 자극 –> encode erythropoietin, vascular endothelial growth factor (VEGF) and nitric oxide synthase
Secondary effects : also mediated by the hypoxia- induced release of vasoactive mediators
– adenosine, endothelin and angiotensin II 등 –> redirect medullary and cortical blood flow
Tertiary effects
– reduction in glomerular filtration rate (GFR) <– vasoconstriction, inflammation and tubular obstruction, epithelial and endothelial injury due to hypoxia and ATP depletion
– increased susceptibility to microvascular thrombosis <– endothelial damage, followed by an inflammatory response involving leukocyte infiltration and the upregulation of chemokines and cytokines in the kidney (–> sterile inflammation)
* NGAL is produced by epithelial cells of the tubule
–> 그래서 primary response to IRI 를 확인하는 marker 로 사용
( rather than a secondary consequence of neutrophil activation )
– IRI 전에 neutrophils 을 제거한다고 해서 IRI 로 인한 Ngal 의 발현증가를 바꾸지 못했다.
– chemotherapeutic immunosuppression 은 원래 AKI 가 없는 환자에서 serum NGAL 을 감소시킨다.
– 하지만 AKI 가 동반된 면역저하 환자에서는 NGAL 을 감소시키지 못했다.
4) Extracellular fluid volume depletion
renin–angiotensin–aldosterone–antidiuretic hormone systems 의 활성화로 신장의 많은 segment 에 영향을 미칠 것으로 예상했지만, 실험결과는 most prominent in the inner medulla 에서만 유전자 발현의 변화가 있었다.
(* inner medulla : final salt and water balances are achieved 되는 곳.)
양에서 ECFV expansion 을 시키면, medullary pO2 를 증가시켰으나, cortical perfusion and pO2 에는 변화가 없었다.
Consequently, the therapeutic value of ECF expansion as a prophylaxis against many different nephrotoxic agents such as radiological contrast material might in part lie in its segment-specific increase in oxygen delivery.
5) Secondary effects of injury
consequence of injury –> hanges secondarily affect multiple areas of the kidney and modulate the patterning
of gene expression
Ex. ) infiltrating leukocytes –> release reactive oxygen species, proteases, elastases and other enzymes –> directly cause tissue damage and change the initial distribution of epithelial responses to tubular injury
–> Capillary occlusion, fibrin aggregation, conversely increased vascular permeability in damaged capillaries
–> Localized oedema in the kidney (encapsulated organ) –> alter transmural pressures –> aggravate venous congestion –> contribute to deteriorating microcirculatory perfusion
–> final outcome of vascular damage : intra- renal shunting and the modulation of perfusion in the renal medulla and cortex
Mitochondrial dysfunction
autopsy samples from patients who died from sepsis
– predominant findings on histopathological examination –> Structural changes in mitochondria
Mouse model (septic AKI) : mitochondrial swelling and disruption of cristae
– Mitochondrial respiratory chain dysfunction –> reduce oxygen utilization –> reduced ATP production –> bioenergetic failure
– PPARγ co- activator-1α (PGC-1α) 또한 sepsis 동안 downregulated 된다. <– major regulator of mitochondrial biogenesis and metabolism
이러한 미토콘드리아의 구조적 변화와 Bioenergetic 변화는 SCr 이 상승하기 전에 발생한다. (injury 자극에 대한 초기 변화이다.)
* Proximal tubule : energy demands 가 높은 부위
* mTAL : 미토콘드리아가 가장 밀집된 nephron segments