CMV Infection (CMV 감염)
- 01_손닥터 의학정보/012_손닥터 감염
- 2018. 7. 15.
CMV Infection
(CMV 감염)
Human cytomegalovirus (HCMV), a betaherpesvirus. HCMV (Human CMV) is the largest member of the human herpesvirus group and is the largest known virus to infect humans. The CMV genome is a linear, double-stranded DNA molecule (236 kbp). CMV double-stranded DNA is wrapped in a nucleoprotein core that is surrounded by matrix proteins and the pp65 antigen of CMV. A lipid envelope that surrounds the matrix and inner core contains viral glycoproteins that are involved in viral entry.

Infection is common in all human populations and reaches 60% to 70% in U.S. cities and nearly 100% in some parts of Africa.
Disease is varied in humans infected with cytomegalovirus (CMV), from no disease in healthy hosts and congenital CMV syndrome in neonates, which is frequently fatal, to infectious mononucleosis syndrome in young adults. In the patient with immunocompromise, CMV produces its most significant and severe disease syndromes in lung, liver, kidney, and heart transplant recipients.
Polymorphonuclear cells, T lymphocytes, endothelial vascular tissue, renal epithelial cells, and salivary glands may all harbor the virus in a nonreplicating or slowly replicating form. Activation from this latent state can occur after immunosuppression, other illness, or the use of chemotherapeutic agents.
The various strains of CMV are species specific and produce a cytopathic effect resulting in greatly enlarged cytomegalic) cells containing cytoplasmic and intranuclear inclusions.
Primary infection occurs in patients who are seronegative and have never been infected with CMV. Secondary infection represents activation of a latent infection or reinfection in a person with seropositive immunity. Clinical CMV disease can result from either primary or secondary infection; in primary infection, virus usually replicates to a higher level and disease is more severe.
The HCMV genome contains a single origin of replication, and like all human herpesviruses, it encodes a DNA polymerase gene and a complete package of genes needed for its own DNA replication. Viral DNA polymerase is an important target for antiviral drugs, and all current therapies for CMV disease inhibit viral DNA polymerase as the final target.5 CMV DNA polymerase is
encoded by a CMV ORF designated UL54. CMV DNA polymerase is highly conserved, with only a 4% occurrence of polymorphic nucleotide variation noted in clinical isolates, and has an important accessory protein, UL44, that enhances the processivity of DNA polymerase. The UL54 and UL44 proteins form the functional complex of complete DNA polymerase in infected cells.
The CMV genome also encodes a protein phosphotransferase enzyme, the product of UL97, whose role in CMV DNA replication is not well understood. The UL97 protein may phosphorylate other proteins involved in DNA replication, and it can phosphorylate CMV UL144. The CMV UL97 protein phosphorylates and inactivates the cellular retinoblastoma tumor suppressor.
진단
Direct detection of antigens in neutrophils with a monoclonal antibody against the CMV matrix protein pp65 has proved particularly useful.
Other methods for detection of CMV DNA or RNA have used labeled viral nucleic acid probes and nucleic acid hybridization in body fluids or tissue specimens.
A polymerase chain reaction (PCR) assay, which uses primers in the gene that encodes CMV immediate early antigen or in the CMV DNA polymerase, has provided a very sensitive technique for detection of CMV. PCR assay can detect small amounts of CMV DNA in many body fluids.
In immunocompetent individuals the presence of IgM and/or low avidity IgG antibodies is a hallmark of primary infection. In the immunosuppressed, however, the antibody rise may be delayed or absent and these tests are at best only of use for retrospective diagnosis. The presence of IgG is used to determine prior exposure of both donors and recipients to CMV infection, most commonly at the time of transplant wait listing, and of donors at the time of organ retrieval but it is of little value after transplantation.
Tests based on the polymerase chain reaction (PCR) carried out on plasma, whole blood or leukocytes are much more sensitive and have become the gold standard in many laboratories in the UK and beyond. Real time PCR offers highly reproducible data on viral load to be obtained rapidly and has become increasing used.
치료
Three antiviral drugs that act to inhibit the viral DNA polymerase have been shown to be effective in the treatment of CMV end-organ disease and have been approved for use in the United States: GCV, foscarnet, and cidofovir (CDV)
(1) Ganciclovir
Ganciclovir, a nucleoside analogue of guanosine and a homologue of acyclovir, was the first antiviral drug shown to be effective in the treatment of CMV disease in humans.
Ganciclovir 의 작용 기전
Ganciclovir 는 CMV 가 감염된 세포에서 UL97 유전자가 만들어 내는 Phosphotransferase 에 의해 Ganciclovir monophosphate 로 전환되고 이 후 세포 효소에 의해 활성형인 Triphosphate 로 전환되어 이것이 바이러스의 DNA 에 결합하여 dGTP 가 결합하는 것을 경쟁적으로 저해함으로써 효과를 나타낸다. 따라서 DNA 복제를 억제함으로써 바이러스 합성을 억제한다. Ganciclovir triphosphate 의 세포내 농도는 Acyclovir 보다 CMV 에 감염된 세포에서 10배 이상 높고, Acyclovir 의 반감기인 1~2시간보다 16.5~24시간으로 훨씬 길기 때문에 CMV infection 에 대해 Ganciclovir 가 효과적이다.
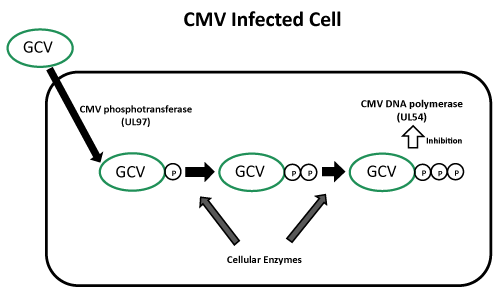
Ganciclovir 에 대한 내성 기전
Ganciclovir 의 인산화에 관여하는 UL97 유전자와 DNA polymerase 유전자인 UL54 의 point mutation 또는 Deletion 에 의해 발생하며, UL97 유전자의 돌연변이가 더 흔하다. (Ganciclovir 에 대한 IC50, inhibitory concentration 이 10 uM 이상의 고도 내성인 경우 (보통 1.5~3 ug/mL 이상일 때를 내성으로 보고), cidofovir 에 교차내성을 보이나, Foscarnet 에는 감수성을 보임.)
Ganciclorvir 내성 발생의 risk factors
(1) 장기간 Ganciclovir 사용
(2) CMV 초감염
(3) ATG 를 포함한 강도 높은 면역억제제의 사용 등
내성검사는 시행이 어려울 뿐 아니라 시험관 내에서 시행한 내성 검사 결과와 약물 치료에 대한 임상적 반응이 반드시 일치하는 것은 아니어서 일반적으로 시행되지는 않는다.
내성을 의심하여 검사를 고려해야 하는 경우
(1) AIDS 환자, 특히 CD4+ 세포수가 50/uL 미만으로 3~4개월 이상 장기간 ganciclovir 치료를 하는 경우
(2) CMV 항체 양성인 공여자로부터 장기 이식을 받은 CMV 음성 수여자에서 장기간 예방적 Ganciclovir 투여시
--> 최대 용량의 항바이러스제를 주사함에도 불구하고 DNAemia 혹은 antigenemia, viremia 가 지속되거나 증가할 때
(3) 조혈모세포 이식 환자에서 CMV 감염에 대해 Ganciclovir 선제 치료를 3주간 시행하였는데, antigenemia 가 계속 증가하거나, 4주간 투여 후에도 음전되지 않고 지속되는 경우
--> 조혈모세포 이식환자에서 ganciclovir 선제치료 2주 후에도 CMV antigenemia 가 계속 증가한다면 ganciclovir 내성보다는 숙주의 면역학적 요인이나 다른 이식 관련 요인들을 먼저 생각해보아야 한다.
내성이 의심될 경우 표현형 검사 (Plaque reduction assay) 와 유전형 검사를 동시에 시행하는 것이 도움이 된다.
Gancyclovir 의 부작용
골수 억제가 가장 중요한 부작용이며, 용량과 연관되어 있다. (호중구 감소, 혈소판 감소 등)
두통, 어지러움, 정신착란, 환시, 정신병, 떨림, 경련, 운동실조, 졸림, 혼수 등이 환자의 5~15% 정도에서 보고됨. 빈혈, 발진, 발열, 간기능장애, 오심, 구토, 호산구 증가 등도 보고됨. 임신 category C 이며, 동물 실험에서 기형 발생등 가능성 있으므로, 임신이나 수유 중에는 사용하지 않는다.
(2) Valganciclovir
VGC is the valine ester of GCV; it has a much greater oral bioavailability than does GCV (about 68% is absorbed compared with 6% to 8% for oral GCV). A valine esterase in the human intestinal mucosa cleaves the valine ester, and GCV enters the bloodstream. Two 450-mg tablets orally result in blood levels that are equivalent to those attained with IV GCV at a dose of 5 mg/kg/day.
(3) Foscarnet
Foscarnet, a pyrophosphate analogue that binds directly to the DNA polymerase of CMV and other herpesviruses, is a reversible competitive inhibitor that does not become incorporated into elongating viral DNA. Foscarnet is associated with significant nephrotoxicity and metabolic toxicity. Renal failure, hypocalcemia, hypomagnesemia, and hypophosphatemia are serious consequences of foscarnet therapy that can be effectively managed through close monitoring of serum creatinine levels, dose adjustment, and replacement of magnesium, calcium, and phosphate losses with oral supplements.
(4) Cidofovir
Cidofovir ([S]-1[3-hydroxy-2(phosphorylmethoxy)propyl] cytokine) is a nucleotide analogue of cytosine that has significant antiviral activity against CMV in vitro.
Cidofovir contains a phosphonate group and does not need to be phosphorylated by a viral enzyme. It is therefore active against thymidine kinase–deficient herpes simplex virus162 and cytomegalovirus, in which mutations in the UL97 gene confer resistance to GCV.
Cidofovir is converted by cellular enzymes to CDV triphosphate, which is the active inhibitor of the viral DNA polymerase. CDV triphosphate has a long intracellular half-life and needs to be given only once weekly. The maximal tolerated dose is 5 mg/kg weekly given intravenously. This dose is given weekly for 2 weeks for induction and then is given once every 2 weeks.
CDV must be administered with oral probenecid (2 g) before each IV dose. The major toxicity of CDV results from its uptake by the proximal convoluted renal tubular cells, which produces degeneration and necrosis of these cells that may be irreversible. Probenecid prevents the uptake of CDV and spares the renal tubular cells from degenerative damage.
Gancyclovir 내성 CMV 감염
내성 검사를 하지 못하는 경우라면 Foscarnet 투여 권장.
UL97 과 UL54 에 모두 돌연변이가 있는 경우라면 ganciclovir 와 cidofovir 에 교차내성을 보이나, UL97 부위에만 돌연변이가 있을 경우 Cidofovir 도 시도해 볼 수 있다. (Ganciclovir 에 대한 IC50, inhibitory concentration 이 10 uM 이상의 고도 내성인 경우 (보통 1.5~3 ug/mL 이상일 때를 내성으로 보고), cidofovir 에 교차내성을 보이나, Foscarnet 에는 감수성을 보임.)
If a clinical isolate of CMV is highly resistant to GCV (IC50 ≥30 μmol/L) and contains mutations in both the UL97 and DNA polymerase genes, cross-resistance to CDV may also be observed. These isolates still remain sensitive to foscarnet.
신이식 환자의 CMV 감염
혈청양성 공여자로부터 혈청음성 환자에게 이식한 경우 CMV 감염의 빈도는 70~90%, CMV 질환의 빈도는 50~60% 로 높다.
Approximately 8% of renal, 29% of liver, 25% of heart and 39% of lung transplants can be expected to experience symptomatic CMV infection
Snydman et al showed a 30% death rate in seronegative recipients of seropositive kidneys who received Anti-Lymphocyte Globulin.
The frequency of disease among the positive recipients of positive kidneys (D+/R+) and positive recipients of negative kidneys (D-/R+) was extremely low.
Because of the multiple human strains of CMV, seropositive organ recipients are at risk of reinfection with a different strain of virus. In this situation, the clinical syndrome is usually less severe than in primary infection and the onset of disease is often delayed to approximately 6-8 weeks post-transplantation.
Seropositive recipients of seronegative grafts (and seronegative blood products) can also develop CMV disease due to reactivation of latent virus. This is usually relatively mild compared to primary infection and also often delayed to approximately 6-8 weeks post-transplantation.
A publication which showed the plot of the probability of CMV disease against viral load in a renal transplant population is instructive. At a viral load of 5 log10 copies per ml the probability was 20%, at 5.5 log10 copies it was 50% and at 6 log10 copies it was about 80%.
Morbidity from CMV is lowest among kidney transplant recipients, but primary infection from a seropositive donor to a seronegative recipient (D+/R−) can occur and is significantly more symptomatic than is secondary infection.
Two studies reported the clinical and laboratory findings from a total of 154 renal transplant patients with CMV infection.
In one of the series, 13 of 18 primary infections were associated with at least two of the following symptoms: fever, leukopenia, atypical lymphocytes, lymphocytosis, hepatosplenomegaly, myalgia, and arthralgia. This constellation of findings has been called the CMV syndrome, which is now defined as CMV infection accompanied by an otherwise unexplained fever of longer than 48 hours, malaise, and a fall in neutrophil count for 3 consecutive days. This most common manifestation of CMV-associated illness in kidney transplant recipients contrasts with the appearance of secondary CMV infections, of which only 19% have been associated with fever.
Clinically significant CMV hepatitis is a rare occurrence among patients after renal transplantation, but elevated hepatic enzyme (aspartate aminotransferase) levels were seen in 10 of 16 cases (63%) of primary CMV infection in kidney transplant recipients. Five cases of CMV interstitial pneumonia were found in this group. Rejection of the transplanted kidney was also observed in 4 of 16 patients with primary infection. In this study, 24 seronegative patients who received kidneys from seronegative donors (D−/R−) and remained seronegative did not undergo rejection. This study is one of the few that reached the conclusion that CMV infection may increase the likelihood of rejection of the transplanted organ. In a large series of 126 kidney transplant patients, hepatic dysfunction was observed in 22%. Severe hepatitis was observed in 7, and CMV was isolated from the bodily fluids of all 7. At autopsy, 5 of these patients had evidence of CMV in the liver.
In a small series of kidney transplant patients in whom CMV pneumonia developed, a mortality rate of 48% was noted. This rate is much less than that observed among bone marrow transplant patients in whom mortality rates as high as 84% have been reported. In kidney transplant recipients who acquire CMV pneumonia, GCV alone has been effective therapy for severe interstitial pneumonia, as was previously noted.
Kidney transplant recipients have also been extensively studied in attempts to prevent CMV disease. In a study of CMV infection, hyperimmune globulin was administered to 24 seronegative kidney transplant recipients within 72 hours of transplantation and was continued for 16 weeks. The rate of CMV infection was 71% compared with 77% in 35 control subjects, but the rate of symptomatic disease decreased from 60% to 21%. This study reported one death from CMV in the treated group and five deaths in the control group; the higher rates of CMV disease and death may have been related to the widespread use of antithymocyte globulin. Prophylaxis with immunoglobulin was not shown to be effective in primary CMV disease after other solid-organ transplants, such as liver transplants.
CMV antigen detection has been used at the start of GCV as preemptive therapy in CMV antibody–positive kidney transplant patients and has met with success in the prevention of CMV disease. Oral GCV therapy has also been successful in lowering CMV DNA levels and in preventing CMV disease after renal transplantation.
끝.
2018. 7. 15 - SJH
'01_손닥터 의학정보 > 012_손닥터 감염' 카테고리의 다른 글
| Fluoroquinolone, Aminoglycoside, Colistin 간단 메모 (0) | 2018.09.23 |
|---|---|
| 쉽게 이해하는 면역학 그림 (0) | 2018.07.17 |
| Cytomegalovirus in Solid Organ Transplantation (장기이식 환자에서 CMV 감염 진료 지침) (2) | 2018.07.15 |
| Sterile pyuria (무균성 농뇨) (0) | 2018.07.15 |
| Infectious mononucleosis (전염 단핵구증) 진단 (0) | 2018.07.15 |


